Eye drop recall
Web A rare strain of bacteria found in recalled eyedrops has been linked to dozens of infections as well as cases of vision loss surgical removal of eyeballs and one. The manufacturer Global Pharma.

Third Eye Drop Brand Recalled One Linked To Serious Infection Vision Loss And A Death Myparistexas
Thirty-five patients were connected to four healthcare.

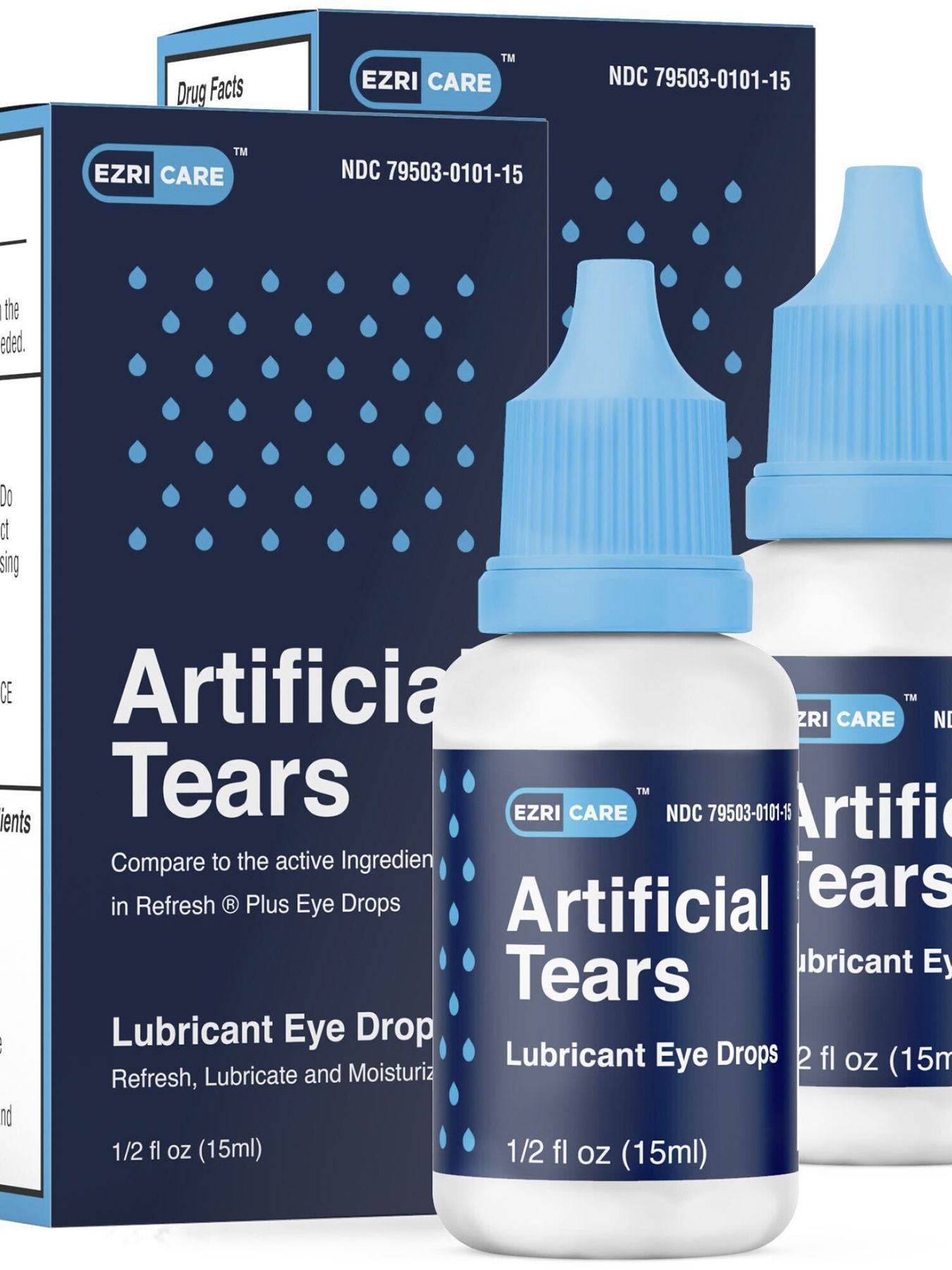
. Web 23 hours agoHealth Manufacturer recalls eyedrops after possible link to bacterial infections Per the CDCs latest update 68 patients across 16 states have been infected. A majority of those affected reported using preservative-free EzriCare. Web CNN Global Pharma Healthcare is issuing a recall of its Artificial Tears Lubricant Eye Drops that were distributed by EzriCare and Delsam Pharma due to.
Web Most cases linked to the outbreak involved eye drops purchased online before the recall. Web The eye drops could be contaminated. This is just the latest over-the-counter eye drop.
Web An alarming outbreak of extensively drug-resistant bacteria linked to eye drops has now sickened 68 people across 16 states according to the latest update from. The drops have not been linked to illness the. Web Two types of artificial tears eye drops have been voluntarily recalled following 55 reports of adverse use effects including eye infections vision loss and even a.
However one reported buying EzriCare at a Costco warehouse. The prescription drops are. Web WASHINGTON US.
Web 4 hours agoPharmedica USA in March recalled Purely Soothing 15 MSM Drops out of concern that the product is not sterile. Web Apotex Corp. Pharmedica is recalling its Purely Soothing 15 MSM Drops.
Health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and. Web A new recall for eye drops has impacted two lots of the Purely Soothing 15 MSM eye drops from Pharmedica USA. Web 18 hours agoUS.
Web The companies involved in the recalls are Phoenix-based Pharmedica and Florida-based Apotex. Initiated a voluntary recall for six lots of Brimonidine Tartrate Ophthalmic Solution on March 1 due to cracks in the caps. Officials are reporting two more deaths and additional cases of vision loss linked to eyedrops tainted with a drug-resistant bacteria.
Web The eye drops were recalled in early February after the Centers for Disease Control and Prevention linked them to an outbreak of Pseudomonas aeruginosa. Web On Thursday the maker of the eyedrops recalled them because of possible contamination. Web The FDA and CDC are advising patients to stop using EzriCare Artificial Tears an over-the-counter brand of eye drops.
Web On March 1 Apotex recalled prescription eye drops used to reduce eye pressure in people with glaucoma or ocular hypertension. Web 22 hours agoEzriCare eye drops had been recalled back in February after being linked to 55 bacterial cases in 12 states. The company recalled six lots.
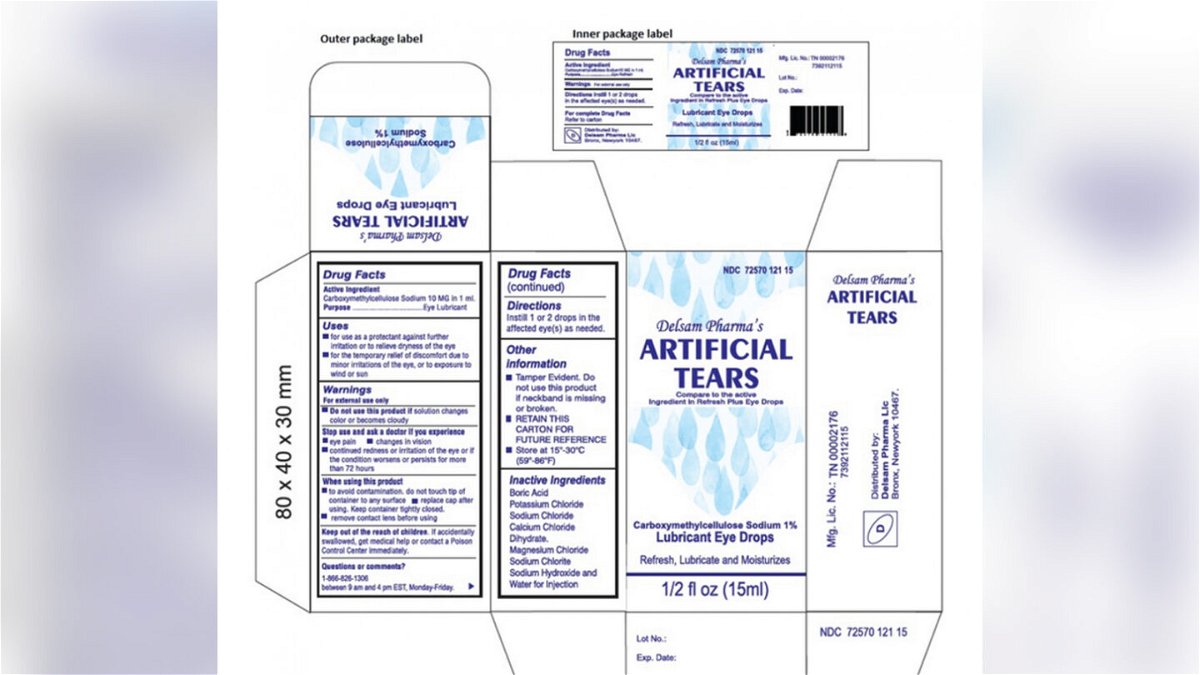
Web Global Pharma Healthcare is voluntarily recalling all lots within expiry of their Artificial Tears Lubricant Eye Drops distributed by EzriCare LLC- and Delsam Pharma to the. Web At the time of the recall there were 55 reports of adverse reactions to the drops including eye infections permanent vision loss and one death from a. If you have questions about the products being recalled you can contact EzriCares distributor at 518-738-7602 or.
O N2vpc3liz1vm

Expire Tag Fees Eye Drop Recall Did Whoopi Fart

Fda To Recall 2 More Eyedrop Brands Due To Contamination Risks Pbs Newshour

Eye Drops Recalls 2023 Products Recalled Because They May Not Be Sterile

Eye Drops Recalled By Two Companies Over Safety Concerns Wsj
Eye Drop Recall 2023 Here S What You Need To Know About The Flurry Of Eye Drop Recalls Cbs News
Eye Drops Recall At Walmart Cvs Walgreens

Fda Eye Drops Recall Lawyer Contaminated Artificial Tears Attorney Hastings Law Firm Medical Malpractice Lawyers

Blackburnnews Com Health Canada Recalls Eye Drops Over Ingredients Not Labeled
Eye Drop Manufacturer Issues Recall Amid Cdc Investigation Of Infections Death Ktvz

Ezricare Eye Drops Recall Linked To Infections Blindness Death

Eyedrops Linked To Vision Loss Infections Have Been Recalled
Pharmasave Advanced Relief Eye Drops Recalled After Packaging Error Poses Health Risks Nwonewswatch Com

Eye Drops Recalled Over Potential Life Threatening Infections

Fda Expands Recall Of Eye Drop Products After Reports Of Eye Infections
:max_bytes(150000):strip_icc()/8-best-eye-drops-for-allergies-of-2023-tout-1bfb54eb47dc4b30930c5666601e0cac.jpg)
Recalled Eye Drops Linked To Infections Vision Loss

South Florida Medical Company Recalls Eye Drops For Glaucoma Miami Herald